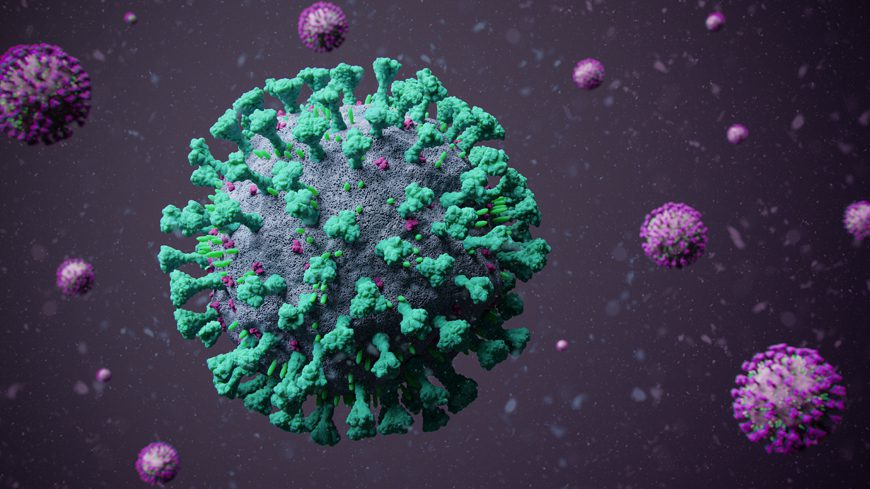
The fight with SARS-CoV-2 is not over yet, and we’re still trying to get it under control. Scientists do whatever they can. They have managed to simulate the transition of the SARS-CoV-2 spike protein structure from when it gets to the host cell.
This study shows that a structure with sugar molecules on the spike protein is actually essential for cell entry. Breaking that structure could actually help us halt the transmission.
So how do people get infected?
One of the most essential things about SARS-CoV-2 is its ability to attach so easily to host cells and transfer its genetic material. This happens through its spike protein. This spike protein is made out of three components: a transmembrane bundle that helps the spike gets attached to the virus and two S subunits on the exterior of the virus. So in order to infect someone, S1 binds to a molecule on the surface of the human cell called ACE2. S2 detaches and then fuses the virus with the human cell membranes.
“Most of the current SARS-CoV-2 treatments and vaccines have focused on the ACE2 recognition step of virus invasion, but an alternative strategy is to target the structural change that allows the virus to fuse with the human host cell. But probing these intermediate, transient structures experimentally is extremely difficult, and so we used a computer simulation sufficiently simplified to investigate this large system but that maintains sufficient physical details to capture the dynamics of the S2 subunit as it transitions between pre-fusion and post-fusion shapes,” stated co-author José N. Onuchic.
Scientists are very interested in the impact of sugar molecules on the spike protein. These sugar molecules are called glycans. To see if it plays an important role, no matter how many, what kind, and what’s the position of glycans, researchers make thousands of simulations by using an all-atom structure-based model.











Leave a Reply